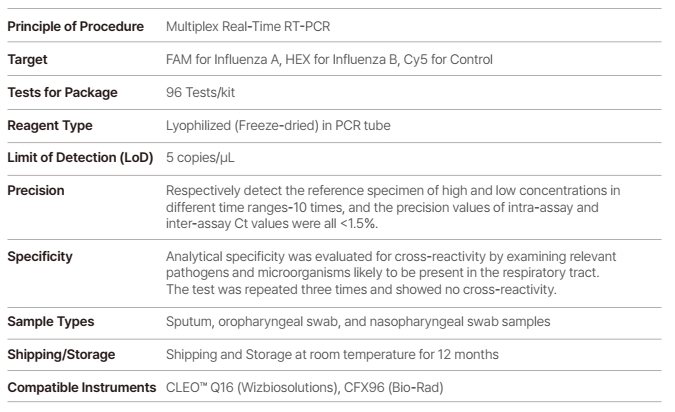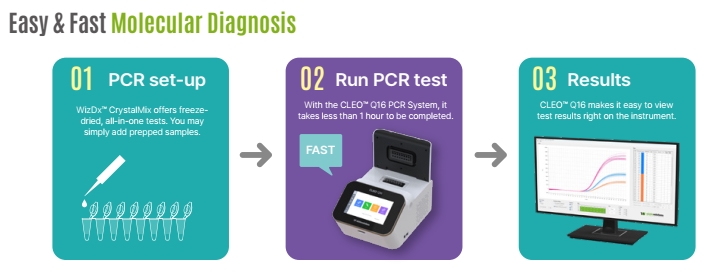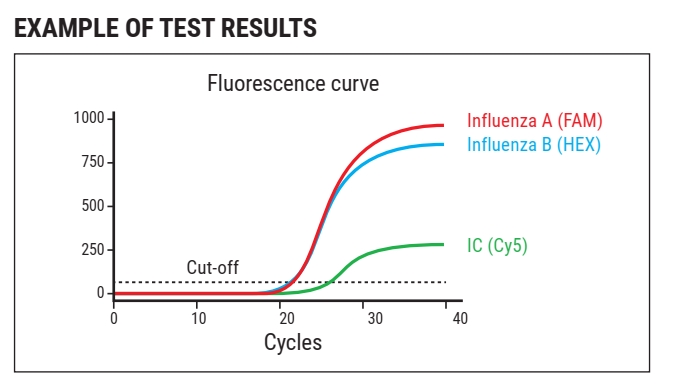


WizDx™ Influenza A/B CrystalMix PCR Kit is intended to be used to achieve qualitative detection of Influenza A/B viral RNA extraction from nasopharyngeal swabs, oropharyngeal swabs, and sputum from patients.
MFDS License No. : IVD-21-300
Download