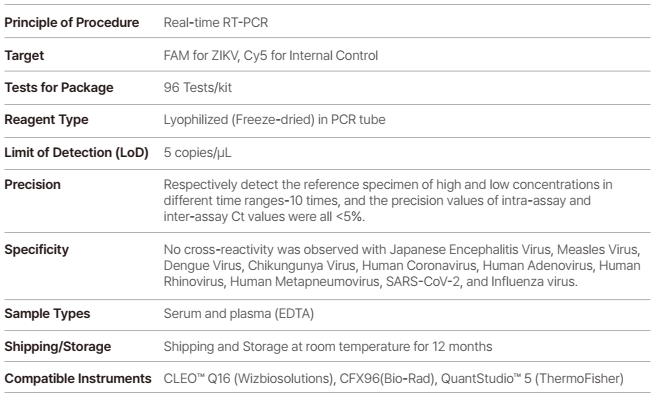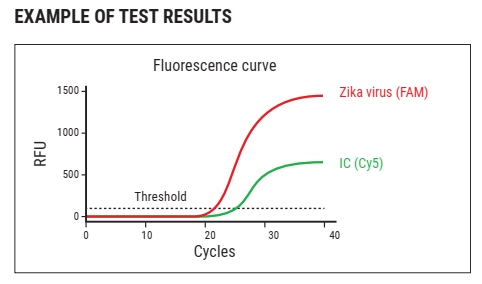

WizDx™ ZIKV CrystalMix PCR Kit is an in vitro nucleic acid amplification assay for the qualitative detection of Zika virus RNA in human blood, serum, or plasma (EDTA) from Zika virus-infected individual specimens using a Real-Time PCR System.
MFDS License No. : IVD-22-6
Download